Mild to moderate pain
Adult: 100-200 mg tid.
Oral
Acute migraine attacks
Adult: 200 mg when 1st symptoms appear, may be repeated after 1-2 hr, if necessary.
|
Indications and Dosage
Oral
Mild to moderate pain Adult: 100-200 mg tid. Oral Acute migraine attacks Adult: 200 mg when 1st symptoms appear, may be repeated after 1-2 hr, if necessary.
|
|
Renal Impairment
Severe: Avoid.
|
|
Hepatic Impairment
Severe: Avoid.
|
|
Administration
Should be taken with food. Take w/ or immediately after meals.
|
|
Contraindications
Hypersensitivity to aspirin or other NSAID. Active or history of GI bleeding or ulceration, severe heart failure, history of GI bleeding or perforation related to previous NSAID therapy. Severe renal and hepatic impairment. Pregnancy (3rd trimester).
|
|
Special Precautions
Patient w/ or w/ history of bronchial asthma, uncontrolled HTN, CHF, established ischaemic heart disease, peripheral arterial disease, cerebrovascular disease, coagulation defects, SLE, connective tissue disorders, history of GI disease (e.g. Crohn’s disease, ulcerative colitis). Hepatic and renal impairment. Pregnancy and lactation.
|
|
Adverse Reactions
Dysuria esp in males; tremor, euphoria, fatigue, pulmonary infiltration, nausea, vomiting, diarrhoea, flatulence, constipation, dyspepsia, abdominal pain, melaena, haematemesis, ulcerative stomatitis, exacerbation of Crohn’s disease and colitis, gastritis, pancreatitis; oedema, HTN, cardiac failure; nephritis, hepatitis, jaundice, abnormal liver function; visual disturbances, optic neuritis, headache, paraesthesia, depression, confusion, hallucinations, tinnitus, vertigo, tremor, dizziness, malaise, drowsiness; thrombocytopenia, neutropenia, agranulocytosis, anaemia.
Potentially Fatal: Exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis; GI bleeding, ulceration or perforation. |
|
Patient Counseling Information
This drug may cause dizziness, drowsiness, fatigue and visual disturbances, if affected, do not drive or operate machinery.
|
|
Monitoring Parameters
Monitor renal function.
|
|
Overdosage
Symptoms: Headache, nausea, vomiting, epigastric pain, GI bleeding, diarrhoea, disorientation, excitation, coma, drowsiness, tinnitus, fainting, convulsions. Renal failure and liver damage may occur in cases of significant acute poisoning. Management: Symptomatic treatment. May administer activated charcoal (or perform gastric lavage for life-threatening situation) w/in 1 hr of ingestion.
|
|
Drug Interactions
Increased risk of bleeding when used w/ corticosteroids, anticoagulants, SSRIs and antiplatelets. Increased risk of nephrotoxicity w/ ciclosporin, diuretics and tacrolimus. May decrease therapeutic effect of antihypertensive agents, diuretics and mifepristone. May exacerbate cardiac failure, reduce GFR and increase plasma glycoside levels. May increase plasma concentration of lithium and methotrexate. Increased risk of developing convulsions when used w/ quinolone antibiotics. Increased risk of haematological toxicity w/ zidovudine.
|
|
Action
Description: Tolfenamic acid inhibits prostaglandin and leukotriene synthesis. It has anti-inflammatory, analgesic and antipyretic effects.
Pharmacokinetics: Absorption: Readily absorbed from the GI tract. Bioavailability: 85%. Time to peak plasma concentration: Approx 60-90 min. Distribution: Distributed into breast milk (small amounts). Plasma protein binding: 99%. Metabolism: Metabolised in the liver. Excretion: Via urine (approx 90%, as glucuronic acid conjugates) and faeces (approx 10%). Plasma half-life: Approx 2 hr. |
|
Chemical Structure
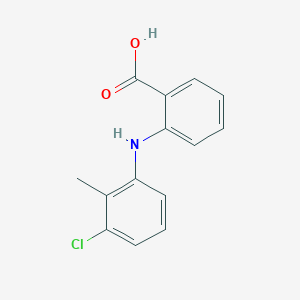 Source: National Center for Biotechnology Information. PubChem Database. Tolfenamic acid, CID=610479, https://pubchem.ncbi.nlm.nih.gov/compound/Tolfenamic-acid (accessed on Jan. 23, 2020) |
|
Storage
Store below 25°C.
|
|
ATC Classification
M01AG02 - tolfenamic acid ; Belongs to the class of non-steroidal antiinflammatory and antirheumatic products, fenamates.
|
|
References
Buckingham R (ed). Tolfenamic Acid. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 21/07/2016. Joint Formulary Committee. Tolfenamic Acid. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 21/07/2016.
|